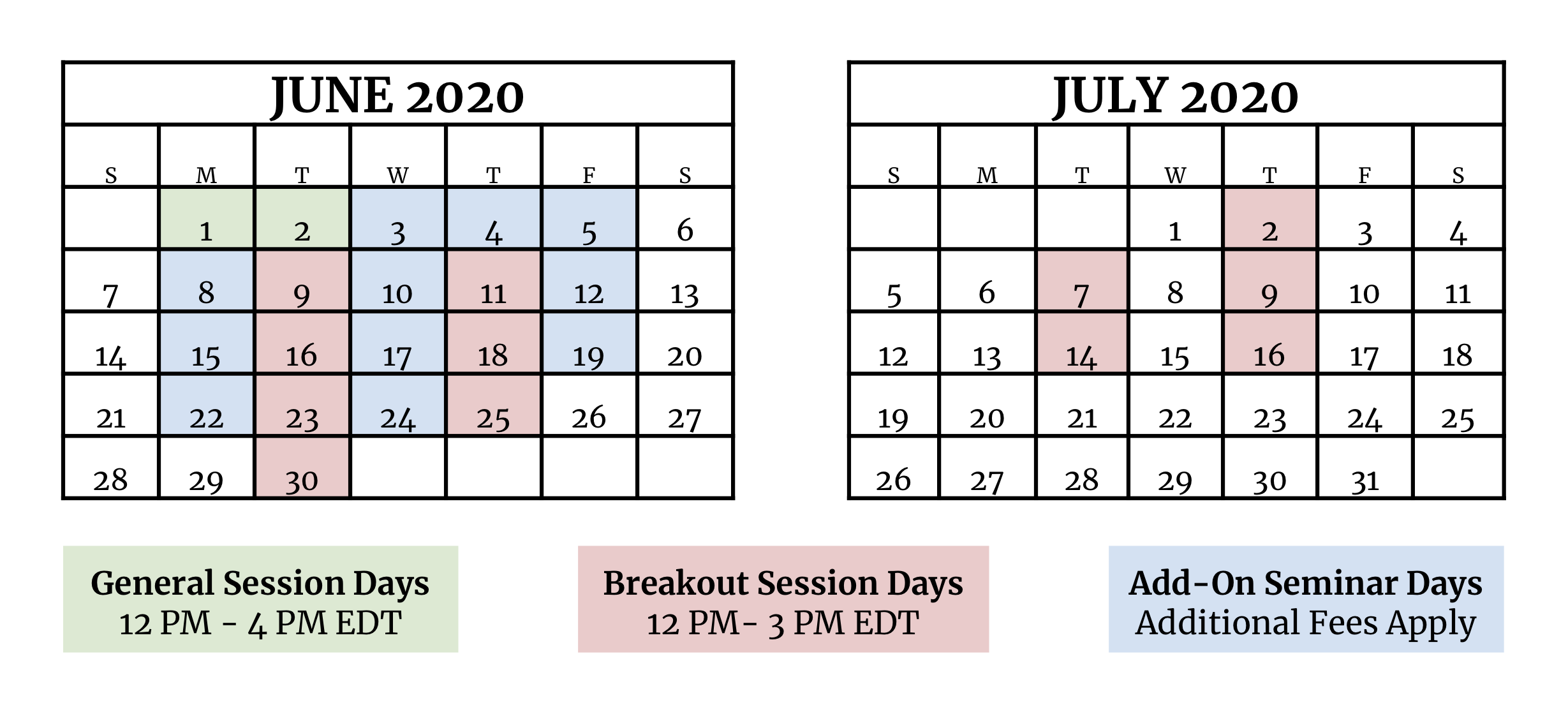
COVID-19 Impact - Prime Label hosted a virtual conference this year, modifying our dates to be more conducive to stay-at-home work schedules. Our 7 general sessions took place over 2 half-days, but our 35 breakout topics and 8 add-on seminars were scheduled sequentially over 6 weeks in June and July. This allowed conference participants to attend over 40 hours of premium regulatory content (with eligible continuing education (CEU) credit) in just the main conference alone.
empty box
empty box
Monday June 1
12:00 pm
1:00 pm
2:00 pm
3:00 pm
Tuesday June 2
12:00 pm
1:00 pm
2:00 pm
3:00 pm
empty box
Tuesdays & Thursdays, June 9 - July 16, 12:00 pm - 3:00 pm
empty box
See breakout descriptions in section below
empty box
empty box
empty box
June 3, 12 - 3p
June 4 & 5, 12 - 3p
June 8, 12 - 3p
June 10 & 12, 12 - 3p
June 15, 12 - 3p
June 17 & 19 & 22
12 - 2p
2 - 4p
June 24, 12 - 3p
European Union Labeling (and Brexit) - What's Next? ** NEW
Canadian Labeling ** (two 3 hour sessions)
Latin and South American Labeling ** NEW
School Lunch (CN) Labeling ** (two 3 hour sessions)
Label Graphics: from NFPs to Names to Claims, and more **
USDA Labeling Basics ** (three 2 hour sessions)
Labeling and Marketing Claims ** NEW (three 2 hour sessions)
Food Service, HRI, and Not-For-Retail Labeling ** NEW
empty box
* all times are Eastern Daylight Time
** optional; additional fees apply
empty box
empty box
empty box
empty box
The Future of Quality: COVID-19 and Beyond...
Tom Chestnut, Sr. VP Global Food Division, NSF
This session will provide an opportunity to learn about some of the latest front line digital food safety trends, such as the use of smartglass technology and AI to detect and correct human error in real time - bringing food safety execution to the next level. Tom Chestnut, Sr. Vice President of NSF International’s Global Food Division will explore what is fueling the trends and how companies are using technology vs.people and travel in the era of coronavirus.
empty box
FTC Labeling Enforcement Priorities
Elizabeth Sanger, Bureau of Consumer Protection, Federal Trade Commission
Novel claims, advertising, and products often face scrutiny by the Federal Trade Commission. Understand how regulators approach new trends and hear an important update regarding FTC enforcement priorities and government intent regarding new rules.
empty box
The Next Horizon - BE Disclosure and Online Compliance
Moderator: Jesse Zuehlke, PhD, Prime Label Consultants
Explore the intersection of two top priorities for many in the regulatory community. Planning is well underway for on-pack and on-line disclosure of Bioengineered Foods, and the explosion of digital marketing and e-commerce continue to tax compliance teams. Learn best practices and reg flags to watch for in your own BE & online labeling implementation.
empty box
Pandemic Panel - Best Practices and Lessons Learned
Moderator: Jesse Zuehlke, PhD, Prime Label Consultants
The COVID-19 Pandemic has had unprecedented impact on business around the globe. A panel of experts across the supply chain will share how their businesses were impacted and critical changes they made to survive in the new normal going forward.
empty box
USDA Labeling & Regulations Update with Q & A
Members of the Labeling and Program Delivery Staff, FSIS, USDA
Over the past year, the USDA has been busy proposing and evolving guidance on GMO, animal production claims and generic label approval. This session will provide an update regarding USDA’s new and pending policies and government intent regarding new and proposed rules. USDA staff labeling experts will also respond to the audience’s food and nutrition labeling questions, and clarify labeling gray areas and unwritten rules.
empty box
Office of Field Operations - FSIS Enforcement
Philip Bronstein, Assistant Administrator, Office of Field Operations, FSIS, USDA
Learn how the Office of Field Operations is guiding inspection activity and USDA enforcement priorities through their district offices around the country. Understand how inspection has proceeded through the COVID-19 pandemic, and what future plans might include. Walk through what the USDA is thinking and how the inspectors make the decisions they do.
empty box
Legal Trends and Challenges in Food Labeling
Robert Hibbert, Partner at Morgan Lewis and Tony Pavel, Senior Food Lawyer, Cargill
This year the food industry is challenged to balance regulatory and marketplace demands in an increasingly litigious environment. This session will give a legal perspective and risk assessment of new regulations, food standard controversies, marketplace trends and technologies, and assess the initiatives of the new administration, three years in.
empty box
Private Label & Small Group Networking
Various Moderators - TBD
Network with industry experts, Private Label teams and conference attendees during a virtual networking hour at the end of the General Session. We plan to have a variety of different small group affinity topics to choose from.
empty box
empty box
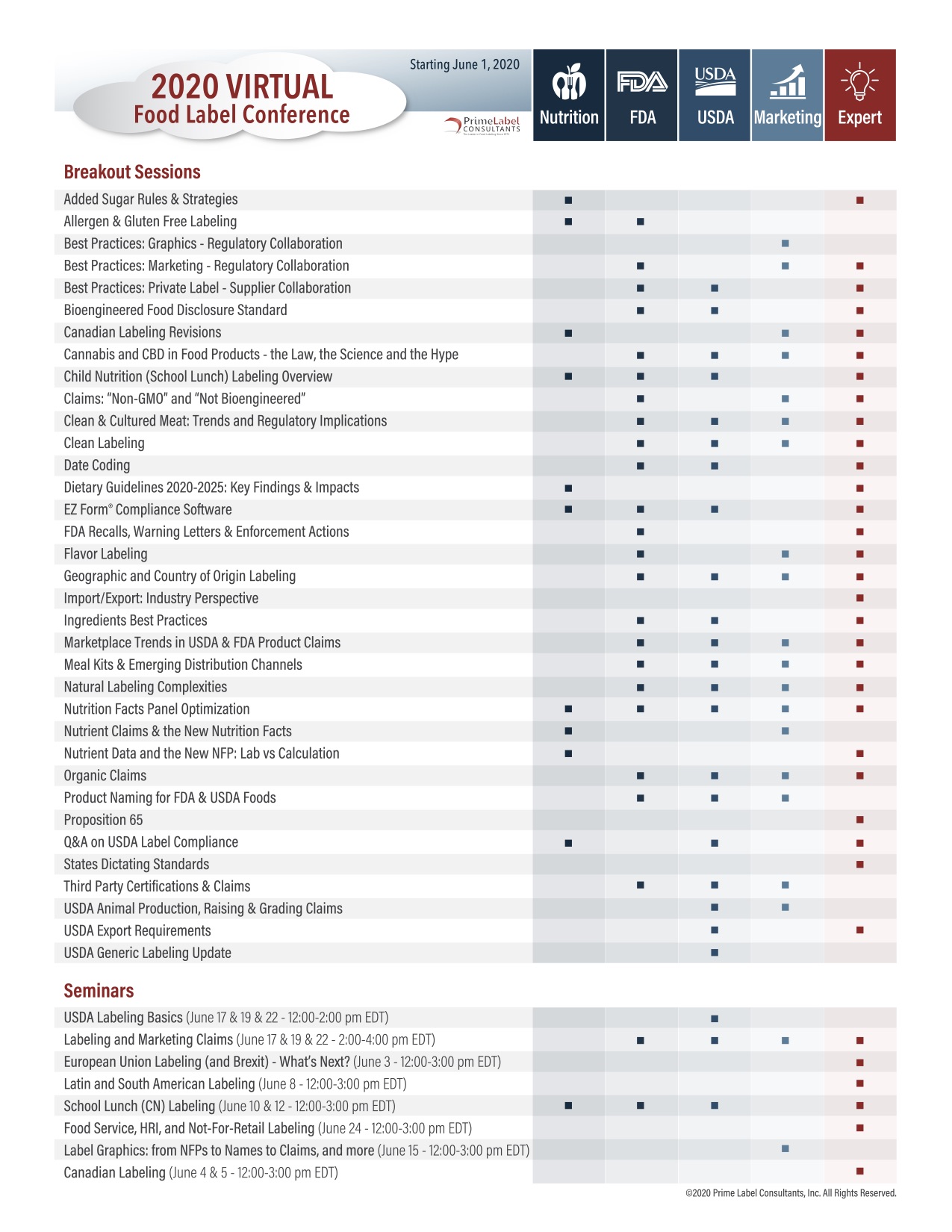
Added Sugar Rules & Strategies
Tracks: Nutrition, Expert
Instructor: Lisa Weller Ashton, MS, Prime Label Consultants
The definition of Added Sugar has been vastly debated since the publication of FDA’s final rule for nutrition labeling. Since then, the agency has continued to release additional guidance to facilitate implementation of the rules. This session will use examples and case studies to explain the FDA’s definition of Added Sugars, propose best practices for troubleshooting data collection, and highlight strategies for optimizing nutrition facts panel sugar declarations.
empty box
Allergen & Gluten Free Labeling
Tracks: Nutrition, FDA
Instructor: Fernanda Almeida, Nestle
Discover the different requirements of the USDA and FDA for labeling allergens, the major source of recalls in the United States. Learn when allergens need to be declared and when they don’t, including the subtleties of containing statements. Dive into the regulations on gluten-free labeling and some considerations of its pros and cons.
empty box
Best Practices: Graphics - Regulatory Collaboration - NEW
Tracks: Marketing
Instructor: Brian DiCrescenzo & Brittany LaSota, Topco Associates LLC
With the proliferation of new claims and regulatory requirements, collaboration between regulatory and graphic design is a huge challenge. This session will cover best-practices for working together and communicating, and troubleshooting when issues arise.
empty box
Best Practices: Marketing - Regulatory Collaboration
Tracks: Marketing, FDA, Expert
Instructor: Jennifer Kiebles, McCain Foods & Caitlin Diederich, Prime Label Consultants
Turn conflict into creative compliance and position products in the best light. This session is intended to explore best practices for marketing and regulatory teams to fulfill a shared goal – to ensure that products are in compliance while keeping them competitive in the marketplace.
empty box
Best Practices: Private Label - Supplier Collaboration
Tracks: FDA, USDA, Expert
Instructor: Amy White, Walmart & Jared Bock, Reser's Fine Foods
With rapid growth in Private Label, Brand Owners, as well as Manufacturers, face a unique challenge to collaborate extensively on label claims and compliance in a rapidly shifting regulatory landscape. Explore best practices for coordination and conflict resolution across the supply chain.
empty box
Bioengineered Food Disclosure Standard
Tracks: FDA, USDA, Expert
Instructor: Trevor Findley, Food Disclosure and Labeling, USDA, AMS
For the first time in the US, industry will need to disclose food's bioengineered BE status (containing BE ingredients) on packages. This session will cover the specifics of the new rule, the options for disclosing this information and the deadline the industry must meet for compliance with this new law.
empty box
Canadian Labeling Revisions
Tracks: Nutrition, Marketing, Expert
Instructor: Michelle Anstey, NSF International
Understand new Canadian nutrition and ingredient labeling regulations and learn how they differ from labeling in the US, including the new Nutrition Facts Table format, ingredient list changes, and allergen declarations. Discuss the subtleties of formulating products for Canada and some of the special issues that apply. Understand what resources are available to you to ensure your Canadian labels are compliant.
empty box
Cannabis & CBD in Food - the Law, the Science and the Hype
Tracks: Marketing, FDA, USDA, Expert
Instructor: Jonathan Havens, Saul Ewing Arnstein & Lehr LLP
Cannabidiol (CBD) is the newest food craze. Find out why CBD-infused products could hold both promise and peril, what we know about the science behind the hype, and what remains to be seen. Learn about the confusing legal and regulatory framework surrounding CBD, and the rules of the road about adding it to food products.
empty box
Child Nutrition (School Lunch) Labeling Overview
Tracks: Nutrition, FDA, USDA, Expert
Instructor: Trish Tung-Tayman, Child Nutrition Labeling Program Office, USDA
Discover the ins and outs of Child Nutrition labeling from the administrators themselves. Explore how CN labeling works and its advantages. Learn about the Agricultural Marketing Service (AMS) quality control program and the CN label application process. Find out where to obtain information and discuss some frequently asked questions.
empty box
Claims: "Non-GMO" and "Not Bioengineered" - NEW
Tracks: Marketing, FDA, Expert
Instructor: John Dillard, OFW Law
With mandatory Bioengineered labeling effective in 2020, this session will explore consumer, regulatory, and legal aspects of a Non-GMO claim vs a Not Bioengineered claim, important considerations for each, and whether there is a difference for your products.
empty box
Clean & Cultured Meat: Trends and Regulatory Implications
Tracks: Marketing, FDA, USDA, Expert
Instructor: Brian Sylvester, Foley & Lardner LLP
Clean meat is meat that is grown in laboratories from animal cells and is born out of a desire to find environmentally friendly alternatives to animal husbandry. It is still in development, but start-ups working on it say it could be coming within the year. This session will explore the consumer drivers, technologies and regulatory impact of cell-based agriculture.
empty box
Clean Labeling
Tracks: Marketing, FDA, USDA, Expert
Instructor: Kantha Shelke, Corvus Blue, LLC
There is no regulatory definition for clean labeling. But the truth is, consumer demand for easy-to-understand ingredient statements and trustworthy labels isn't going anywhere. When it comes to meeting what consumers want, it is a lot easier said than done. This session will cover the technical realities associated with the concept of a “clean” label from an R&D and Regulatory perspective.
empty box
Date Coding - NEW
Tracks: FDA, USDA, Expert
Instructor: Janis Hochstetler, Iowa Department of Agriculture
What does that EXP 1/31/2025 really mean on that can of soup? This session will examine terms, consumer expectations, industry standards, FDA and USDA regulatory requirements, safety vs quality, shelf life studies and resources all relating to food code dating.
empty box
Dietary Guidelines 2020-2025: Key Findings & Impacts - NEW
Tracks: Nutrition, Expert
Instructor: Sarah Levy, FoodMinds
Explore the latest status of the 2020 Dietary Guidelines Advisory Committee, including their Scientific Report. Review new conclusions and recommendations, and how the 2020-2025 Dietary Guidelines for Americans will impact consumer trends and your own product marketing and labels.
empty box
EZ Form Compliance Software
Tracks: Nutrition, FDA, USDA, Expert
Instructor: Fred Mosher, Prime Label Consultants
Discover how this powerful regulatory software can streamline your labeling process by providing required forms, labels and Nutrition Facts Panels, updated for the new rule. Learn how EZ Form's smart checks and record keeping can save you time and increase your compliance. Get access to EZ Form's programmer to answer your advanced questions, and learn tips and tricks for optimizing this powerful label compliance tool.
empty box
FDA Recalls, Warning Letters & Enforcement Actions
Tracks: FDA, Expert
Instructors: Alexandre Gapihan & Maria Kalousi-Tatum, Morgan, Lewis & Bockius LLP
FDA has multiple initiatives for monitoring manufacturer compliance and communicating violations of FDA regulations. Learn more about key tools available for compliance including blockchain technology, trends in enforcement, best practices for preparing for and responding to recalls and the impact of recalls on brand integrity.
empty box
Flavor Labeling
Tracks: Marketing, FDA, Expert
Instructor: Lisa Cummins, FONA International Inc.
Flavor labeling tops the charts as one of the most common complicated aspects of product labeling. This session will cover flavor descriptors and the laws governing labeling and specific situations including food allergens, flavoring agents, ingredient classifications, flavor categories, organic, kosher and halal.
empty box
Geographic and Country of Origin Labeling - NEW
Tracks: Marketing, FDA, USDA, Expert
Instructor: Katelyn Hilferty, Morgan Lewis & Bockius LLP
Can you claim that your product or ingredients are from a specific location? When is that required? Understand how to apply geographic origin and style claims, and learn when you must include country of origin information.
empty box
Import/ Export: Industry Perspective - NEW
Tracks: Expert
Instructor: Jen Dadak, Campbell Soup Company
Understand the regulatory complexity and best practices for navigating the global supply chain and international distribution. Hear about compliance, knowledge management, and business impacts from a multinational CPG.
empty box
Ingredients Best Practices - NEW
Tracks: FDA, USDA, Expert
Instructor: Justin Crews, J & J Snacks
Learn best practices for building an ingredient statement on your product labels: choosing a common and usual name, interpreting your supplier's specification, identifying flavors and colors, as well as important aspects of documentation and record-keeping.
empty box
Marketplace Trends in USDA & FDA Product Claims
Tracks: Marketing, FDA, USDA, Expert
Instructor: Julie Luebbert, Ambassador Meat Distributors, Inc
Learn about the latest innovations in USDA and FDA product labeling trends. Discuss the consumer drivers behind these trends and how the food industry is responding. Delve into the subtleties of the newest food claims and explore alternatives to satisfy market demand.
empty box
Meal Kits & Emerging Distribution Channels
Tracks: Marketing, FDA, USDA, Expert
Instructor: Tom Hofer, PurFoods, LLC
Meal kits, direct-to-consumer, and online marketplaces are increasingly popular. Without a clear definition or agency jurisdiction, industry has been left to fill in the gaps while regulators have been playing catch-up. This breakout will discuss recent regulatory guidance on meal kit labeling requirements, the retail store exemption and the interaction of USDA, FDA, state and local regulations on these products.
empty box
Natural Labeling Complexities
Tracks: Marketing, FDA, USDA, Expert
Instructor: Evangelia Pelonis, Keller and Heckman LLP
Understand the ins and outs of Natural labeling. Explore the differences between FDA and USDA definitions of “Natural”. Discuss the impact on retail, restaurants and customer perception, and explore the pros and cons of this contentious claim.
empty box
Nutrition Facts Panel Optimization
Tracks: Nutrition, Marketing, FDA, USDA, Expert
Instructor: Christina Bechtold, CEO, Prime Label Consultants
Nutrition Label Reform has upended two decades of NFP/packaged product optimization and revealed new labeling challenges with serving size changes and the new mandatory dual column requirement. Food companies are back at the drawing board to synchronize their nutrition labeling with their target markets. This case study driven session will illustrate how nutrition takes center stage when implementing the new rules and opportunities to mitigate negative impact.
empty box
Nutrient Claims & the New Nutrition Facts
Tracks: Nutrition, Marketing
Instructor: Caitlin Diederich, Prime Label Consultants
Understand the FDA and USDA requirements for this well-defined set of claims and how the new NFP rule will impact them. Dive into expressed versus implied claims, such as Lean, Low Fat, Sodium, and Omega claims, amongst others. Explore relative claims and Front of Package nutrient claims and the subtleties of their different requirements.
empty box
Nutrient Data and the New NFP: Lab vs. Calculation
Tracks: Nutrition, Expert
Instructor: Amy Hiller, Eddy Packing Company, Inc.
Explore the integrity of the Nutrition Panel by understanding the differences between calculated and analytical methods of producing nutrient data. Discuss the various sources of nutrient data, and issues of nutrient variability. Understand how the new NFP rule will impact lab analysis and your record-keeping systems.
empty box
Organic Claims
Tracks: Marketing, FDA , USDA, Expert
Instructor: Janel Abro, NSF International
Explore the intricacies of Organic claims, including the certification process, documentation, types of claims and the subtleties of this labeling.
empty box
Product Naming for FDA & USDA Foods
Tracks: FDA , USDA, Marketing
Instructor: Lisa Weller Ashton, MS, Prime Label Consultants
Explore USDA and FDA regulations on product naming and standards of identity. Find out the difference between common and usual, descriptive, standard, and fanciful names and what conventions are required for each. Learn about the components of product names, including many types of qualifiers.
empty box
Proposition 65
Tracks: Expert
Instructor: Michael Geibelson, Robins Kaplan LLP
For products sold in the state of California, compliance with the Safe Drinking Water and Toxic Enforcement Act of 1986, or Proposition 65, is essential to mitigate litigation risk. Come and learn the important challenges to the published list of substances, recent changes to the clear and reasonable warnings requirements, and how your food or packaging may be impacted.
empty box
Q&A on USDA Label Compliance
Tracks: Nutrition, USDA, Expert
Instructor: Callie Kambanis, Prime Label Consultants
Explore the ins and outs of USDA labeling - such as product names and qualifiers; undefined RACC and nutrient claims; negative, uncured, organic and natural claims. Bring your specific questions on the subtleties of labeling to our industry and PLC experts.
empty box
States Dictating Standards - NEW
Tracks: Expert
Instructor: Ann Begley, Morgan, Lewis & Bockius LLP
State efforts to regulate the labeling of food products have exploded with at least half the states pursuing legislation in this area. In response, organizations are suing the states claiming free speech protections and competitive disadvantages. In this session, a legal expert will address current state law activities, the status of lawsuits & federal preemption.
empty box
Third Party Certifications & Claims
Tracks: FDA, USDA, Marketing
Instructor: Jim Riva, Where Food Comes From, Inc.
With more and more consumers demanding independent validation, third party certification (including gluten free, non-GMO, process verification, and ingredient quality claims) is a growing trend. The session will provide an overview of how third party certification programs work, how to find a provider, and manage the process. Learn which claims can be certified and what common certification criteria looks like. Discuss best practices for a certification audit.
empty box
USDA Animal Production, Raising & Grading Claims
Tracks: USDA, Marketing
Instructor: Savannah Bland, Eddy Packing Company, Inc.
Discuss the complexity of Animal Production and Breed Raising claims such as Cage-Free Poultry, Free-Range, No Added Hormones or Antibiotics, Humanely Raised, Certified Angus and others. Find out the latest in USDA grading claims.
empty box
USDA Export Requirements
Tracks: USDA, Expert
Instructor: James Chisholm, Import/Export Policy Development Staff, USDA
Discover the latest in USDA export requirements, including the intricacies of customs documentation, online resources & systems, and the new PHIS export module. This session covers USDA export processes, it does not cover individual country's labeling requirements.
empty box
USDA Generic Labeling Update
Tracks: USDA
Instructor: Debbie Nece, Cargill Protein
Find out the latest enforcement priorities, requirements and best practices for the expanded generic rule. Explore generic eligibility and compliance, and discuss enforcement subtleties with FSIS. Understand what you need to do to keep thorough records and discuss strategies to reduce risk.
ATTENDEE TITLES
REPRESENTATIVE COMPANIES
Corporate Marketing Manager
Registered Dietitian Nutritionist
Director of Regulatory & Labeling
Executive Brand Manager
Regulatory & Nutrition Manager
Labeling Specialist
International Marketing Specialist
Graphic Engineer
Document Control Administrator
Global Product Compliance Mgr
HACCP Coordinator
Executive Director of R&D
Menu Labeling Manager
Laboratory Director
General Counsel
Director of Quality Assurance
Labeling & Nutrition Program Leader
Manager - Graphic Process
Chief Science Officer
New Product Manager
Food Technologist / Scientist
Food Safety Manager
R&D-Tech Services
Chief Flavor Officer
Regulatory Affairs Project Leader
Label & Claim Substantiation Mgr
Technical Services Supervisor
USDA Food Technologist
Vice President of Operations
Chief Quality Officer
Abbott Nutrition
ADM / Wild Flavors
Advanced Food Systems
AdvancePierre
Agra Informa
Ajinomoto Windsor
Albertsons/Safeway
Amazon.com
Ambassador Meat
Ameriqual Foods
Athens Foods
ATK Foods
Bellisio Foods
Berks Packing
Bernardi Italian Foods
Boar's Head
Bob Evans Farms
Bob's Red Mill
Buddy's Kitchen
Burke Corporation
Butterball
Campbell Soup
Cargill
Cavendish Farms
Classic Foods
Columbus Foods
ConAgra Foods
Congressional Offices
Cooper Farms
Creminelli Fine Meats
CTI Foods
Cuisine Solutions
Custom Culinary
Custom Made Meals
Damascus Bakery
Diestel Turkey Ranch
Dunkin Brands
EAS Consulting Group
Ed Miniat
Eddy Packing
Engelhart Gourmet
FDA/CFSAN
Flying Food Group
FoodMinds
Fresh and Easy
Fresh Creative Foods
Fresh Mark
Frisch's Restaurants
Frontera Foods
Frozen Specialties
FTC
Gamay Food Ingredients
General Mills
Gerber
Giorgio Foods
Givaudan
Flavors
GNP Company
Golden State Foods
Griffith Laboratories
Hans Kissle
Harry and David
H-E-B
Hillshire Brands
Hormel
Hot Mama's Foods
Illes Seasonings & Flavors
Indiana Packers
Int'l Flavors & Fragrances
ILSI
InterTribal Buffalo Council
J & J Snack Foods
Jack Link Snacks
Jennie-O Turkey
JMH International
Johanna Foods
Johnsonville Sausage
Jones Dairy Farm
JRS USA
JTM Food Group
Kayem Foods
Kellogg Company
Kerry
Ketchum
Keystone Foods
King Arthur Flour
Koch Foods
Kraft Heinz
Krogers
Label Insight
Lifespice Ingredients
Little Lady Foods
Lopez Foods
Lower Foods
Luvo
Malt-O-Meal
Maple Leaf Farms
Maple Leaf Foods
McCain Foods
McDonald's
McKee Foods
MegaMex Foods
Meijer
Monogram
Morgan Foods
Morgan Lewis & Bockius
National Beef
Next Phase
Nestle
Newly Weds Foods
Niman Ranch
Noodles & Company
NAMI
Nosherei
NSF International
Oberto Brands
Organic Valley
Overhill Farms
Pacific Natural Foods
Paige Food Service
Perdue Foods
Phillips Resources
Pilgrim's Pride
Pinnacle Foods
Plumrose USA
Politico
Publix Super Markets
Quaker Maid Meats
Ready Pac Foods
Reinhart Foodservice
Request Foods
Reser's Fine Foods
Rich Products
Ruiz Food Products
Sanderson Farms
Sargento Foods
Schwan's
Seaboard Foods
Sensient Flavors
Settlers Jerky
SGK
sgsco
Skyline Chili
Smithfield Foods
Sopakco Foods
Southeastern Mills
Stampede Meat
Subway
Sun-Maid
Supervalu
Sysco
Target
Taylor Farms
The Suter Company
Topco
Trader Joe's
Twinings
Tyson Foods
Unilever
USDA/AMS
USDA/FSIS
Vantage Foods
Vaughan Foods
Vienna Beef
Wayne Farms
Wegmans Markets
Wellshire Farms
Where Food Comes
Whitsons Culinary
Whole Foods Market
Wild Flavors
Windsor Foods
Wisdom Natural
* Preliminary list of speakers & moderators - typically we have over 50 professionals from government and industry presenting topics at the Food Label Conference.
Elizabeth Sanger, Bureau of Consumer Protection, FTC
Richard Boyd, USDA-AMS, Child Nutrition Division
Trish Tung-Tayman, USDA-AMS, Child Nutrition Division
Rosalyn Murphy-Jenkins, Director of LPDS, USDA-FSIS
Jeff Canavan, Deputy Director of LPDS, USDA-FSIS
Philip Bronstien, Assistant Administrator, USDA-FSIS
Tammie Ballard, USDA-FSIS
Beth McKew, USDA-FSIS
Melinda Mallon, USDA-FSIS
Tawana Harrington, USDA-FSIS
Janice Fabina, USDA-FSIS
Kierra Lucas, USDA-FSIS
Janice Watkins, USDA-FSIS
Jessica Forshee, PhD, IEPDS, USDA-FSIS
Robert Hibbert, Morgan Lewis & Bockius LLP
Ann Begley, Morgan Lewis & Bockius LLP
Katelyn Hilferty, Morgan Lewis & Bockius LLP
Ryan Fournier, Morgan Lewis & Bockius LLP
Tony Pavel, Cargill
Debbie Nece, Cargill
Jen Dadak, Campbell Soup
Kantha Shelke, PhD, Corvus Blue LLC
Patricia Phillips, Phillips Resources
Lisa Cummins, FONA
Tom Hofer, PurFoods, LLC
Sarah Levy, FoodMinds
Jennifer Kiebles, McCain Foods USA
Brian P. Sylvester, Foley & Lardner LLP
Michael Geibelson, Robins Kaplan LLP
John Dillard, OFW Law
Savannah Bland, Eddy Packing Company, Inc.
Phil Daniel, TechLinkUSA
Fabiola Montero, TechLinkUSA
Evangelia Pelonis, Keller and Heckman LLP
James Riva, Where Food Comes From, Inc
Justin Crews, Wellshire Farms
Richard Young, Senior Food Industry Professional
Brittany LaSota, Topco Associates LLC
Brian DiCrescenzo, Topco Associates LLC
Jonathan Havens, Saul Ewing Arnstein & Lehr LLP
Erin Taraborrelli, NSF International
Tom Chestnut, NSF International
Jared Bock, Resers
Jonathan Lackie, Quality Assurance International
Amy Hiller, Eddy Packing Company, Inc.
Janis Hochstetler, State of Iowa
Fernanda Almeida, Nestle
Amy White, Walmart
Christina Bechtold, Prime Label Consultants
Jesse Zuehlke, PhD, Prime Label Consultants
Fred Mosher, Prime Label Consultants
Lisa Ashton, Prime Label Consultants
Caitlin Diederich, Prime Label Consultants
Taylor Hart, Prime Label Consultants
Callie Kambanis, Prime Label Consultants
Celia Yau, Prime Label Consultants
USDA Labeling Basics
Wednesday June 17th & Friday June 19th & Monday June 22nd (12:00 pm - 2:00 pm EDT - 6 hours in three, 2-hour sessions)
Tracks: USDA
Instructors: Lisa Weller Ashton, MS, & Callie Kambanis, Prime Label Consultants
USDA labeling can be very confusing with many different sources for rules, policies, and regulations. Stakes can be high with generic labels no longer having FSIS's stamp of approval. In addition, local inspectors are giving added scrutiny to labels, and the risk of Noncompliance Records is greater than ever. Learn the fundamentals of USDA labeling in this seminar. Our seminar covers:
...and much more!
empty box
Labeling and Marketing Claims - NEW
Wednesday June 17th & Friday June 19th & Monday June 22nd (2:00 pm - 4:00 pm EDT - 6 hours in three, 2-hour sessions)
Tracks: Marketing, FDA, USDA, Expert
Instructor: Jesse Zuehlke, PhD, General Manager, Prime Label Consultants
Ensuring compliance and substantiation of new product claims is challenging. Labeling and marketing claims represent some of the best opportunities to differentiate and promote your product, but there is always a risk of warning letters and other enforcement when not managed correctly. Learn best practices from Prime Label’s visibility into emerging market trends. This session will cover:
...and much more!
This important session is designed to help determine new potential claims for your items, and how to apply them within FDA and USDA requirements.
empty box
European Union Labeling (and Brexit) - What's Next? - NEW
Wednesday June 3rd (12:00 pm - 3:00 pm EDT - one half day)
Tracks: Expert
Instructor: Jackie Healing, NSF International
European Union food labeling is highly regulated and the requirements are set out by European law. Discover the complexities and gain an insight into EU labeling regulations, understanding specifics on complex areas such as claims and mandatory requirements. This seminar will have a focus on recent and future EU developments and the possible implications of Brexit for the UK. Learn and identify key differences between EU and US labelling. This seminar covers:
empty box
Latin and South American Labeling - NEW
Monday June 8th (12:00 pm - 3:00 pm EDT - one half day)
Tracks: Expert
Instructor: Phil Daniel, TechLink International
Mexico has recently updated their food labeling requirements and all food labels need to compliant with the latest requirements by 2021. This is a substantial change where every food item will need to be updated to meet the new standard. This seminar covers:
...and much more!
empty box
School Lunch (CN) Labeling
Wednesday June 10th & Friday June 12th (12:00 pm - 3:00 pm EDT - 6 hours in two, 3-hour sessions)
Tracks: Nutrition, FDA, USDA, Expert
Instructor: Patricia Phillips, Phillips Resources
The Trump administration has recently changed US school lunch requirements. Come learn about these changes as well as get a primer on complicated, highly specialized Child Nutrition regulations. Learn the what, how and why of CN labeling and Product Formulation Statements in this full day seminar, as well as alternatives to a CN label.
Taught by Patricia Phillips of Phillips Resources, this seminar covers:
...and much more!
empty box
Food Service, HRI, and Not-For-Retail Labeling - NEW
Wednesday June 24th (12:00 pm - 3:00 pm EDT - one half day)
Tracks: Expert
Instructor: Lisa Weller Ashton, MS, Prime Label Consultants
Products manufactured for Food Service - hotels, restaurants, and institutions - are not always subject to the same requirements as retail packaged labeling. Labeling for other not-for-retail items can also be a challenge due to limited regulatory information. With a scarcity of agency guidance, this session will review some regulatory requirements, but focus on industry expectations and best practices for HRI, not-for-retail, and other retail distribution channels. This seminar covers:
empty box
Join this half day session to focus on discussion and best-practices for labeling items outside conventional retail sale.
empty box
Label Graphics: from NFPs to Names to Claims, and more
Monday June 15 (12:00 pm - 3:00 pm EDT - one half day)
Tracks: Marketing
Instructors: Brian DiCrescenzo & Brittany LaSota, Topco Associates LLC
FDA and USDA food label regulations have stringent content requirements and must comply with
size, color, language, and location rules. In addition, the new nutrition facts panel is 5% to 100% larger than existing formats,
which creates challenges fitting all label features on a package. In this half day seminar, learn tips and tricks for graphic design of food labels in the United States:
...and much more!
empty box
Canadian Labeling
Thursday June 4th & Friday June 5th (12:00 pm - 3:00 pm EDT - 6 hours in two, 3-hour sessions)
Tracks: Expert
Instructor: Erin Taraborrelli, NSF International
Canada has recently updated its food labeling requirements. In addition, it is up to food manufacturers to ensure
their product labels are compliant before they go to market, and the regulations can be difficult to interpret. Learn
the regulations that govern food labeling in Canada, the differences with US labeling and CFIA enforcement. This seminar covers:
...and much more!
Main Conference: June - July, 2020
$1,495 Regular Registration
$1,695 Late Registration (after May 15)
Add-On Training: June 3 - July 1, 2020
$995/day* (6 hour training)
$695/day* (3 hour training)
*Savings of $300 off the second or more training