
Sponsor Prime Label Consultants' 2025 Food Label Conference and showcase your brand at the premier food and beverage labeling event, taking place June 1-4 in Washington DC. Our 250+ annual attendees represent 7 of the top 10 and about half of the top 100 food manufacturers in the United States. We are also proud to host 9 US agencies, and over 50 government and industry experts speaking on over 40 different labeling topics!
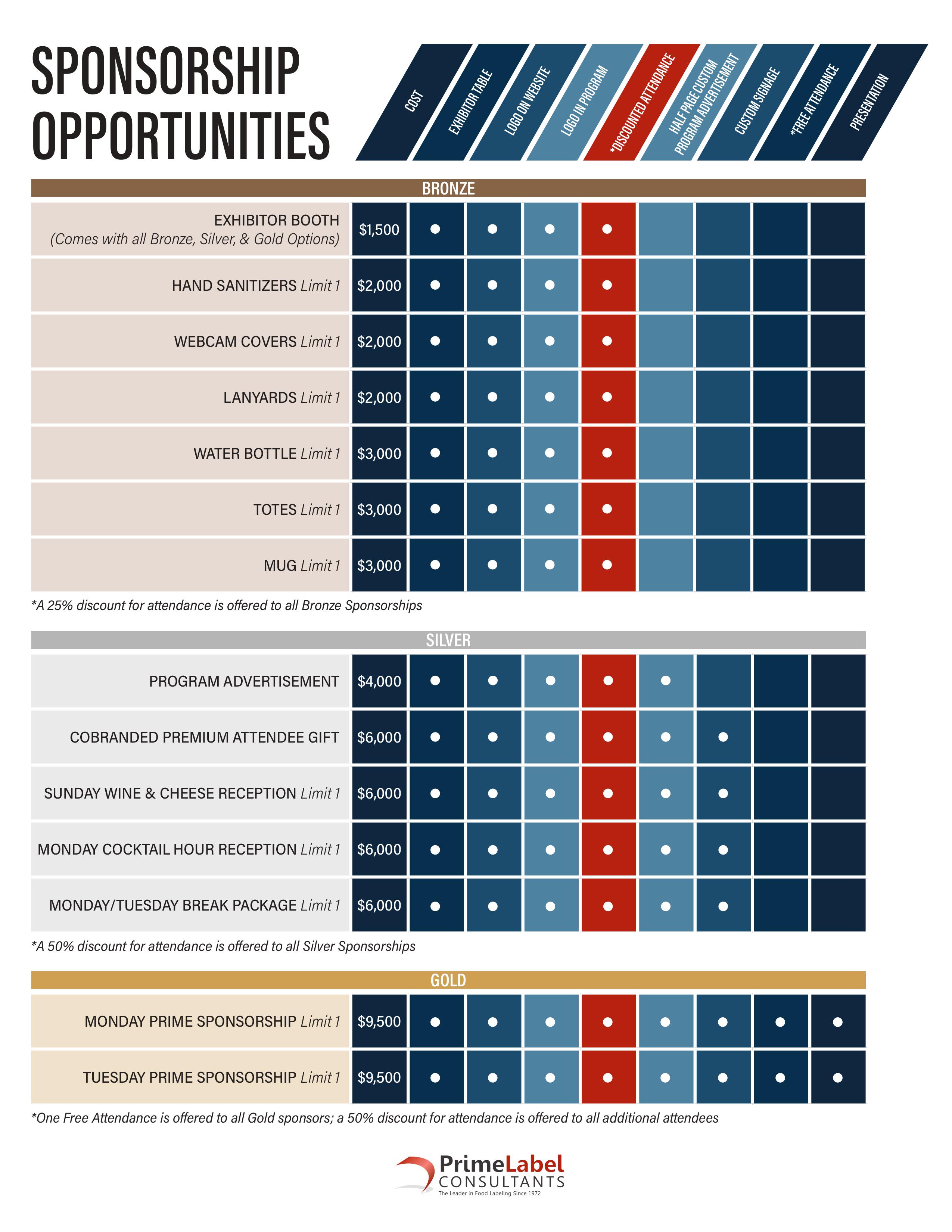
Participate in the Food Label Conference via Bronze, Silver or Gold level sponsorships. All sponsors receive a tabletop exhibit and options to include a wide range of sponsorships including advertisements, branded gifts, and breaks/receptions/day packages. To explore the availability of sponsorship/tabletop packages, please contact Melody Tilford at Melody@PrimeLabel.com or 202.599.4414 ext.702.
Additional Information:
- The tabletops will be available for the entire duration of the Main Conference and will be adjacent to the General Session ballroom (where all meal and networking activities take place).
- A limited number of sponsorships are available on a first come, first serve basis.
- Sponsors can bring materials and signage suitable for a 6 ft skirted tabletop.
- All sponsorship fees must be paid within 30 days of signed agreement and due to custom branding, are non-refundable.
For more information about this year’s sponsorship opportunities, email Melody@primelabel.com
empty box
The Food Label Conference brings together government policy makers and industry experts in a collaborative exchange of trends, ideas and best practices. Our 7 general sessions will take place over 2 half-day, live sessions on June 3-4, 2024. These sessions will highlight current and former government officials giving insight into government priorities and enforcement actions for the upcoming year.
empty box
FDA Regulatory Update *
Speaker TBD
Join us for a critical agency update of the latest FDA guidances, proposed rules, implementation, and food program reorganization. Hear recent updates on guidance statements, proposed rules, and FASTER Act implementation.
empty box
USDA Labeling Policy Update *
Jeff Canavan, Labeling and Program Delivery Staff, FSIS, USDA
A key update from the Labeling and Program Delivery Staff (LPDS) on the new nutrition rule, expansion of generic label approval along with other policy changes, critical insights, and what to expect in the year ahead. Dialog with the labeling regulators for guidance on your most challenging labeling issues.
empty box
Legal Perspective: Updates and Predictions for the Next Year *
Robert Hibbert, Wiley Rein LLP
Tony Pavel, Perfect Day Foods
Two leading DC legal minds share their perspective on expected regulatory changes, emerging trends and risks, food standard controversies, and marketplace trends and technologies.
* sessions subject to change
empty box
empty box
Added Sugar Trends and Case Studies
Lisa Weller Ashton, Food Regulatory Consultant
Tracks: Nutrition, Expert
The definition of Added Sugar has been vastly debated since the publication of FDA’s final rule for nutrition labeling. Since then, the agency has continued to release additional guidance to facilitate implementation of the rules. This session will use examples and case studies to explain the FDA’s definition of Added Sugars, propose best practices for troubleshooting data collection, and highlight strategies for optimizing nutrition facts panel sugar declarations.
empty box
Bioengineered Labeling (NBFDS) Update
Alexandria Fischer, Food Disclosure and Labeling Division, USDA AMS
Tracks: FDA, USDA, Expert
With mandatory bioengineered disclosure in place now for a full year, industry has begun to standardize best practices for labeling. This session will cover any updates and nuances to the specifics of the new rule, the options for disclosing BE status and plans for USDA enforcement.
empty box
Canadian Labeling
Michelle Anstey, NSF International
Tracks: Expert
Understand new Canadian nutrition and ingredient labeling regulations and learn how they differ from labeling in the US, including the Front-of-Package symbol, Nutrition Facts Table and ingredient list formats, and allergen declarations. Discuss the subtleties of formulating products for Canada and some of the special issues that apply. Understand what resources are available to you to ensure your Canadian labels are compliant.
empty box
Cannabis and CBD in Food Products
Jonathan Havens, Saul Ewing Arnstein & Lehr LLP
Tracks: FDA, USDA, Marketing, Expert
Cannabidiol (CBD) remains in the news, especially since the U.S. Food and Drug Administration’s (FDA or the Agency) January 26th announcement that existing regulatory frameworks for foods and supplements are not appropriate for CBD, and that the Agency will work with Congress on a path forward. Despite this, CBD continues to be used in foods and supplements. Find out why CBD-infused products could hold both promise and peril, what we know about the science behind the hype, and what remains to be seen. Learn about the confusing legal and regulatory framework surrounding CBD, when and how the federal landscape in this area might change, and the rules of the road about adding it to food products.
empty box
Carbon Labeling
Amaru Sanchez, Wiley Rein LLP
Tracks: FDA, USDA, Marketing, Expert
Consumers and investors continue to show increasing interest in environmental and sustainability commitments by companies, and the food industry has responded in kind. However, there are few widely accepted industry standards for many environmental claims such as “carbon offset”, “carbon neutral”, and “sustainable,” making these claims an increasingly frequent source of litigation by consumers, interest groups, and regulatory agencies. In this breakout session, the presenter will cover topics such as environmental claims (e.g., carbon offset, sustainable, climate-friendly) as well as the relevant regulatory agencies (e.g., FTC, SEC, FDA, USDA, etc.) involved. Additionally, the presenter will explore the role of certification bodies in this space and relevant scenarios to identify and avoid common pitfalls when making environmental claims.
empty box
Child Nutrition (School Lunch) Labeling Overview
Trish Tung-Tayman, USDA Child Nutrition (CN) Labeling Program Office, USDA-AMS
Tracks: Nutrition, FDA, USDA, Expert
Discover the ins and outs of Child Nutrition labeling from the administrators themselves. Explore how CN labeling works and its advantages. Learn about the Agricultural Marketing Service (AMS) quality control program and the CN label application process. Find out where to obtain information and discuss some frequently asked questions.
empty box
Clean Labeling
Kantha Shelke, Corvus Blue LLC
Tracks: FDA, USDA, Marketing, Expert
There is no regulatory definition for clean labeling. But the truth is, consumer demand for easy-to-understand ingredient statements and trustworthy labels isn't going anywhere. When it comes to meeting what consumers want, it is a lot easier said than done. This session will cover the technical realities associated with the concept of a “clean” label from an R&D and Regulatory perspective.
empty box
Date Coding
Janis Hochstetler, Iowa Meat & Poultry Inspection Bureau
Tracks: FDA, USDA, Expert
What does that EXP 1/31/2025 really mean on that can of soup? This session will examine Terms, Consumer Expectations, Industry Standards, FDA and USDA Regulatory Requirements, Safety vs Quality, Shelf Life Studies and Resources all relating to food code dating.
empty box
Dietary Supplement Labeling Nuances
Erin Taraborrelli, Source Nutraceutical, Inc.
Tracks: Nutrition, Expert
The dietary supplement market is constantly growing and more and more companies are moving into this space. Dietary supplement labeling differs from that of conventional food labeling in what you must include, the claims that are available to you, and other responsibilities for the brand owner. It is In this breakout session we will provide an overview of the regulatory requirements, challenges and common pitfalls in dietary supplement labeling, substantiation and the FDA and FTC’s role in regulating the dietary supplement industry.
empty box
FDA Claims Best Practices/ Workshop
Katy Enright, Mars-Wrigley
Tracks: FDA, Marketing, Expert
Claims Workshop - Join this interactive session to test your knowledge about claims trending in the market and evaluate claim acceptability in a fun way. Bring your smartphone to participate anonymously and enjoy a session without slides!
empty box
FDA Updates: Healthy Claim and FOP Labeling
Mason Weeda, OFW Law
Tracks: FDA, Marketing, Expert
In September 2022 FDA proposed to update the definition of “healthy.” Initially set in 1994, FDA found the current definition does not align with current nutrition science, federal dietary guidelines, and the updated nutrition facts label. This session covers the history of the “healthy” definition; what the proposed rule aims to accomplish; the impact this will have on food labeling; FDA’s next steps; FDA’s related research on a voluntary “healthy” symbol; and FDA’s research on a mandatory “front of pack” (FOP) labeling system.
empty box
Flavor Labeling Trends
Lisa Cummins, McCormick Flavor Solutions | McCormick FONA
Tracks: FDA, Marketing, Expert
Flavor labeling tops the charts as one of the most common complicated aspects of product labeling. This session will cover flavor descriptors and the laws governing labeling and specific situations including food allergens, flavoring agents, ingredient classifications, flavor categories, organic, kosher and halal.
empty box
FTC Advertising & Claims
Liz Sanger, Federal Trade Commission
Tracks: FDA, USDA, Marketing, Expert
Learn about the FTC rules on advertising and how they apply to your food package artwork and claims. Find out what the FTC's enforcement priorities are and learn the subtleties of online marketing and social media.
empty box
Geographic Claims
Jesse Zuehlke, Prime Label Consultants
Tracks: FDA, USDA, Marketing, Expert
Labeling product with origin claims, geographic statements and imagery, or even references to culinary styles can be complex. This session will breakdown the various requirements from FTC and FDA, and recently proposed rulemaking from USDA. Learn important strategies for labeling origin and geographic claims, and understand key risks to watch out for.
empty box
Legal Risks: Demand Letters and Litigation Trends
Ivan Wasserman, Amin Talati Wasserman
Tracks: FDA, Marketing, Expert
Plaintiff litigation and demand letters are becoming one of the primary risks to consider in developing product marketing strategies. Learn how to navigate the shark tank with this discussion of the current litigation landscape, how to best protect your company, and what to do if when your product becomes a target.
empty box
Mandatory COOL Labeling
Sonja Jones, Food Disclosure and Labeling Division, USDA-AMS
Tracks: FDA, USDA, Marketing, Expert
Country of Origin Labeling is managed by the Food Disclosure and Labeling Division of AMS USDA, and required for retail labeling of a range of raw agricultural commodities. Learn how to determine if your products are covered and the labeling requirements, along with common FAQs to help fully implement your labeling strategy.
empty box
Mexican Labeling
Phil Daniel & Fabiola Montero, TechLink International
Tracks: Expert
All Mexican labels were required to meet the latest food labeling requirements by 2021. We will discuss this substantial change and what shifted as a result of the new standard.
empty box
Natural Labeling Complexities
Eve Pelonis, Keller and Heckman LLP
Tracks: FDA, USDA, Marketing, Expert
Understand the ins and outs of Natural labeling. Explore the differences between FDA and USDA definitions of “Natural”. Discuss the impact on retail, restaurants and customer perception, and explore the pros and cons of this contentious claim.
empty box
NFP Calculations: Serving Sizes, DVs & Rounding Nuances
Fred Mosher & Michelle Liang, Prime Label Consultants
Tracks: Nutrition, FDA, USDA, Expert
Explore the integrity of the Nutrition Panel by understanding the differences between calculated and analytical methods of producing nutrient data. Discuss the various sources of nutrient data and how to apply them to determine serving size and daily values. Understand the nuances of FDA rounding rules. Includes a brief introduction to Prime Label’s EZ FormⓇ food labeling software.
empty box
Nutrient Content Claims
Caitlin Diederich, Prime Label Consultants
Tracks: Nutrition, Marketing
Learn about FDA and USDA requirements for expressed and implied claims including common call-outs such as Low Calorie, Low Fat, No Added Sugar and High Protein. Explore relative claims and front of pack nutrient claims and the subtleties of their different requirements.
empty box
Organic Labeling Update and Q&A
Erin Healy, USDA AMS National Organic Program
Tracks: FDA, USDA, Marketing, Expert
2023 marks what many are calling the most significant new rulemaking from USDA on the Organic Program since its inception. Learn about recently-published rules, including Strengthening Organic Enforcement, and how the changes may impact your own organic operations and labels.
empty box
Plant-Based and Substitute Proteins: Naming and Regulatory
Brian P. Sylvester & Jessica O'Connell, Covington & Burling LLP
Tracks: FDA, Expert
As plant-based foods continue to gain ground in the U.S. marketplace, questions around lawful product naming and claims have captured the interest of FDA, stakeholders and Congress culminating most recently in FDA's publication of its Draft Guidance on plant-based milk alternatives, a particularly popular category of plant-based foods. This session will cover FDA's Draft Guidance, providing the legal POV on insights not just for plant-based milks, but more broadly for understanding how FDA will approach the issue of naming of plant-based alternatives more generally. We will also consider this draft guidance in the broader context of consumer scrutiny and litigation and share our views on best practices for managing risk for a range of plant-based foods and alternative protein products.
empty box
Proposition 65
Michael Geibelson, Robins Kaplan LLP
Tracks: Expert
For products sold in the state of California, compliance with the Safe Drinking Water and Toxic Enforcement Act of 1986, or Proposition 65, is essential to mitigate litigation risk. Come and learn the important trends in claims, developments in court decisions and the clear and reasonable warnings requirements, and how your food or packaging and exposure to class action litigation may be impacted.
empty box
Q&A on FDA Compliance
Jesse Zuehlke, Caitlin Diederich & Marissa Hagedorn, Prime Label Consultants
Tracks: Nutrition, FDA
Find out the answers to those nagging questions about your FDA labels. From product naming to claims and allergens, bring your specific questions to be answered by a PLC Consultant.
empty box
Q&A on USDA Compliance
Emily Hendricks, Prime Label Consultants
Tracks: Nutrition, USDA
Explore the ins and outs of USDA labeling - such as product names and qualifiers; undefined RACC and nutrient claims; negative, uncured, organic and natural claims. Bring your specific questions on the subtleties of labeling to our industry and PLC experts.
empty box
Sustainability & Environmental Marketing Claims
Sam Jockel & Rachel Lowe, Alston & Bird
Tracks: FDA, USDA, Marketing, Expert
Environmental claims are now ubiquitous in the marketplace and we’ve all seen a marked increase in litigation. Marketers are facing risk from regulators, NGOs, consumer class action attorneys, and competitors, even when they have vetted their sustainability-related product labeling and advertising. Marketers are also bracing for an update to FTC’s Green Guides governing environmental marketing claims. This session will cover regulatory and litigation developments, including recent court rulings and provide insights on risk mitigation.
empty box
USDA Animal Production, Raising & Grading Claims
Emily Hendricks, Prime Label Consultants
Tracks: USDA, Marketing, Expert
Discuss the complexity of Animal Production and Breed Raising claims such as Cage-Free Poultry, Free-Range, No Added Hormones or Antibiotics, Humanely Raised, Certified Angus and others. Find out the latest in USDA grading claims.
empty box
USDA Generic Approval
Celia Yau & Kirby Ham, Prime Label Consultants
Tracks: USDA, Expert
Find out the latest enforcement priorities, requirements and best practices for the expanded generic rule. Explore generic eligibility and compliance, and discuss enforcement subtleties with FSIS. Understand what you need to do to keep thorough records and discuss strategies to reduce risk.
empty box
USDA Nutrition Library & FoodData Central
Kyle McKillop, Food for Health of People and the Environment Lab, USDA
Tracks: Nutrition, FDA, USDA, Expert
Hear from USDA to better understand USDA FoodData Central. In 2019 USDA instituted the most significant changes to 35 years of content in the Standard Reference nutrition libraries. Learn about the new structure, partner industry data, and how to use this public database.
empty box
USDA Product Trends
Debbie Nece, Cargill Protein
Tracks: USDA, Marketing
Dive into the latest product name and claims trends in USDA labeling. Presented in a case study format, this session will facilitate discussion of trends, marketing innovations, and gray areas to be aware of.
Office of Congresswoman Rosa DeLauro, CT
Office of Congresswoman Alma Adams, NC
Office of Congresswoman Grace Meng, NY
Trish Tung-Tayman, USDA-AMS, Child Nutrition Division
Erin Healy, NOP Division Director, NOP, USDA-AMS
Rosalyn Murphy-Jenkins, Director of LPDS, USDA-FSIS
Jeff Canavan, Deputy Director of LPDS, USDA-FSIS
Tammie Ballard, USDA-FSIS
Melinda Mallon, USDA-FSIS
Tawana Harrington, USDA-FSIS
Janice Fabina, USDA-FSIS
Kierra Lucas, USDA-FSIS
Cindy Watkins, USDA-FSIS
Hannah Filloon, USDA-FSIS
Sally Jones, USDA-FSIS
Beth McKew, USDA-FSIS
Paul Wolseley, USDA-FSIS
James Chisholm, IEPDS, USDA-FSIS
Robert Hibbert, Wiley Rein LLP
Tony Pavel, Perfect Day Foods
Kantha Shelke, PhD, Corvus Blue LLC
Brian P. Sylvester, Covington & Burling LLP
Patricia Phillips, Phillips Resources
Lisa Cummins, FONA International Inc.
Phil Daniel, TechLinkUSA
Joe Baumert, Director of F.A.R.E
Evangelia Pelonis, Keller and Heckman LLP
Sarah Levy, FoodMinds
Mason Weeda, OFW Law
Erin Taraborrelli, NSF International
Michelle Anstey, NSF International
Christina Bechtold, Prime Label Consultants
Jesse Zuehlke, PhD, Prime Label Consultants
Fred Mosher, Prime Label Consultants
Lisa Weller Ashton, Prime Label Consultants
Emily Hendricks, Prime Label Consultants
Celia Yau, Prime Label Consultants
* Preliminary list of speakers & moderators - typically we have over 40 professionals from government and industry presenting topics at the Food Label Conference.
PLC’s Private Label Partnership Program was created to maximize opportunities for Brand Owners to connect face-to-face with Private Label Suppliers at the annual Food Label Conference, the leading event for labeling and compliance professionals. We are excited to welcome a number of Brand Owners at this year's event. Stay tuned....
empty box
Main Conference: June 2 - 3, 2025
Food Label Conference sponsors receive discounted attendance.
- A 25% discount for attendance is offered to all Bronze Sponsorships
- A 50% discount for attendance is offered to all Silver Sponsorships
- One Free Attendance is offered to all Gold sponsors; a 50% discount for attendance is offered to all additional attendees
Interested in registering multiple people? Contact Melody@primelabel.com to learn more about group discounts.
Please use this address to ship sponsorship materials:
GRAND HYATT WASHINGTON
Attn: Prime Label Consultants
1000 H Street NW
Washington, D.C. 20001
SHIPPING GUIDELINES:
• Packages should arrive no earlier than Saturday, June 1st and no later than Tuesday, June 4th.
• Include the total number of boxes shipped on your label (ex: 1 of 3).
• Include the sponsor name on the package, in the return address field.
• Please bring your package tracking number(s) with you in case there are any problems.
• You can ship your items back through the hotel at the FedEx Business Center, located one floor above the Food Label Conference.
If you have questions about logistics, please contact Melody@primelabel.com

Grand Hyatt Washington DC
1000 H Street NW Washington, D.C., USA, 20001
+1 202 582 1234 grandwashington.hyatt.com
Reservations: Please click here to register for PLCs' exclusive discount on rooms for the conference. This deal expires May 10, 2025 or when the room block is full. Don't miss out on discounted rates!
Directions: Conveniently located at Metro Center - on the Red, Orange, Blue & Silver lines.
ATTENDEE BENEFITS
ATTENDEE TITLES
REPRESENTATIVE COMPANIES
Manager of Regulatory Affairs
Labeling Specialist
Registered Dietitian
Food Scientist
Regulatory & Documentation Specialist
Director - Quality and Food Safety
Specification & Compliance Manager
Senior Counsel
Research and Development
Director Marketing/New Products
CEO/ President
QA Supervisor
HACCP and Labeling Coordinator
Technical Services Manager
Marketing Manager
Packaging and Compliance Specialist
Document Management Specialist
Director - Technical Services
Plant Operations Manager
Director of Food Labeling
Director of Food Safety and Quality
Staff Attorney
Managing Partner
Director - Foods and Supplements
Sr. VP Business Development & Finance
Product Development Manager
Scientific & Regulatory Affairs Director
Nutrition & Regulatory Specialist
Senior Director - Nutrition
Food Safety Manager
Ahold Delhaize
Albertsons/Safeway
Alston & Bird LLP
Amazon.com
Ambassador Meat
American Soy Prod.
Amin Talati Wasser.
Applegate
At Last Gourmet
Aunt Millie's Bakeries
Bar-S Foods a Sigma
Bell & Evans
Bellisio Foods
Bonduelle Fresh
Brakebush Brothers
Burke Corporation
Campbell Soup
Cargill
Chelten house
CJ Foods
CK Development Serv.
Clemens Food group
Columbus Craft meats
Cooper Farms
Covington & Burling
CTI Foods
Custom Culinary
Custom Made Meals
Direct Plus Deli Group
Don Lee Farms
Eddy Foods
FDA
FTC
Flagstone Foods
FONA International
FSIS/ USDA
FoodMinds
FoodNavigator-USA
Foodspace Tech.
FoodTrition Solutions
Fresh Mark, Inc.
Gamay Food Ing.
General Mills
Gerber Poultry
Godshall's Meats
Great Lakes Cheese
Greenleaf Foods
Griffith Foods
H-E-B
Harry and David
Home Market Foods
Hy-Vee Fresh Comm.
IHS Markit
Illes Seasonings
Indiana Packers Corp.
Iowa Meat & Poultry Inspection Bureau
John Soules Foods
Johnsonville
JPG Resources
JSL Foods, Inc.
JTM Food Group
Kellogg Company
Kerry
Keuirg/Dr. Pepper
Kiolbassa Provision
Kraft Heinz
Krogers
Label Compliance Sol.
Pilgrim's Pride Co.
Pillers Fine Foods
Plenus Group, Inc.
Pulmuone USA
Label Insight
Publix Super Markets
Lamb Weston
Litehouse Inc.
LKK (USA) Foods
Lopez Foods, Inc.
LPDS/ USDA
Lyons Magnus
Maple Leaf Farms
McCain Foods
McDonald's
Medifast
Meijer
Michael Foods
Mistica Foods LLC
Monogram Foods
Morinaga America
Nardone Bros Baking
Nation Pizza & Foods
NOP / USDA
Nestle
New Seasons Market
Newly Weds Foods
NAMI
NSF International
Oberto Brands
Orca Bay Foods
OSI Group, LLC
Pacific Inter. Marketing
Palermo's Pizza
Patterson TMP
Peapod Digital Labs
Peco Foods, Inc.
Pederson's Natural
Rembrandt Foods
Renaissance Food Grp
Request Foods, Inc.
Riba Foods
Reser's Fine Foods
Rich Chicks
Richelieu Foods, Inc.
Russ Davis Wholesale
Sanderson Farms
Saratoga Food
Sargento Foods
Seaboard Foods
Sigma Foods
Simmons Prep. Food
SK Food Group
Smithfield
Sovos Brands
Specialty Foods Group
Starbucks Coffee
Sun-Maid
Sweet Candy Co.
Swift Prepared Foods
Sysco
Tall Tree Foods
Target
Taylor Farms
TechLink International
TH Foods
Classic Jerky Co.
The Original Cakerie
The Suter Company
Trader Joe's
TreeHouse Foods
US Foods
Vanee Foods
Walgreens
Walmart
Wayne Farms
WG Provisions
World Variety Produce
Whole Foods Market